Exploring the Mysteries of the Smallest Particle of an Element: An In-Depth Look at the Atom
The world of particle physics is a fascinating realm where scientists delve into the intricacies of matter and its fundamental building blocks. At the heart of every element lies the smallest particle of an element known to us – the atom. In this article, we embark on a journey to unravel the secrets of the atom, exploring its structure, properties, and the groundbreaking discoveries that have shaped our understanding of the microscopic world.
I. The Birth of the Atom: A Historical Perspective
The concept of the atom dates back to ancient Greece, where philosophers such as Democritus proposed the idea of indivisible particles that make up matter. However, it wasn’t until the 19th century that scientific evidence began to accumulate, thanks to the work of pioneers like John Dalton and Dmitri Mendeleev.
II. Atomic Structure: Breaking It Down
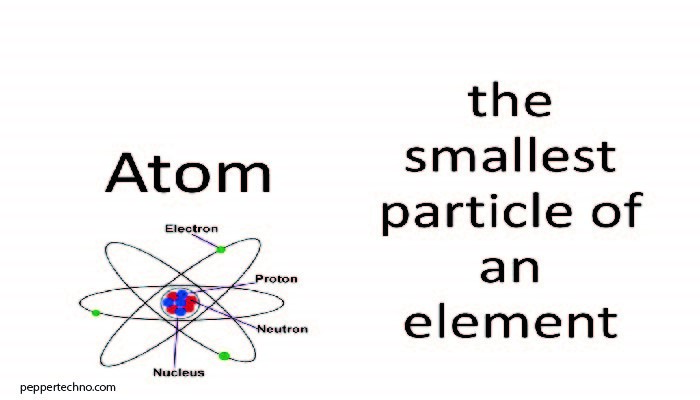
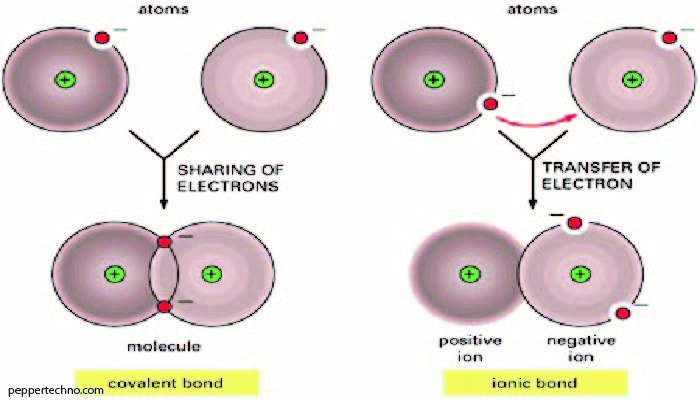
The atom, as we know it today, consists of subatomic particles with distinct properties. At its core is the nucleus, comprised of protons and neutrons, while electrons orbit in shells surrounding the nucleus. The balance of these particles determines an element’s unique characteristics.
III. Protons: Positive Charges at the Core
Protons, positively charged particles within the nucleus, play a crucial role in defining an element’s identity. The number of protons in an atom’s nucleus is its atomic number, distinguishing one element from another on the periodic table.
IV. Neutrons: The Silent Stabilizers
Neutrons, electrically neutral particles found alongside protons in the nucleus, contribute to the atom’s mass without affecting its charge. They serve as stabilizers, preventing the positively charged protons from repelling each other due to their like charges.
V. Electrons: Dancing Around the Nucleus
Electrons, negatively charged particles, orbit the nucleus in specific energy levels or shells. Their movement within these shells gives each element its distinct chemical properties. The arrangement of electrons is crucial in determining an element’s behavior during chemical reactions.
VI. Quantum Mechanics: The Quantum Leap in Understanding Atoms
In the early 20th century, the advent of quantum mechanics revolutionized our understanding of atomic behavior. Pioneering scientists like Niels Bohr and Erwin Schrödinger introduced mathematical models that described the probability of finding electrons in specific regions around the nucleus, challenging classical notions of deterministic physics.
VII. Quantum Numbers and Atomic Orbitals
Quantum numbers dictate the distribution of electrons within an atom. These numbers, including principal, azimuthal, magnetic, and spin quantum numbers, define the unique characteristics of atomic orbitals. Understanding these quantum principles is crucial for comprehending the intricacies of atomic structure.
VIII. Subatomic Particles Beyond the Basics
The exploration of subatomic particles goes beyond protons, neutrons, and electrons. Scientists have discovered particles like quarks and leptons, which constitute the building blocks of protons, neutrons, and electrons. The world of particle physics continually expands as researchers probe deeper into the mysteries of these fundamental entities.
IX. The Standard Model: Unifying Particle Physics
The Standard Model of particle physics serves as the cornerstone of our understanding of subatomic particles. This theoretical framework encapsulates the electromagnetic, weak, and strong nuclear forces, providing a comprehensive explanation of the behavior of particles within the universe. However, it also leaves room for unanswered questions, such as the nature of dark matter and dark energy.
X. Advanced Techniques in Particle Physics
Modern technology has empowered scientists to study subatomic particles with unprecedented precision. Accelerators like the Large Hadron Collider (LHC) at CERN enable researchers to recreate extreme conditions similar to those moments after the Big Bang, offering insights into the fundamental nature of matter.
XI. Applications of Atomic Understanding
Beyond theoretical exploration, our understanding of the smallest particles has practical applications. Fields such as medicine, materials science, and technology leverage this knowledge to develop new technologies, diagnostic tools, and materials that enhance our daily lives.
Conclusion
The smallest particle of an element, the atom, is a complex and dynamic entity that has intrigued scientists for centuries. From the early conceptualizations of ancient philosophers to the cutting-edge research in contemporary particle physics, our understanding of the atom has evolved dramatically. As we continue to explore the mysteries of the smallest particle, we unlock the secrets of the universe and pave the way for groundbreaking advancements in science and technology.